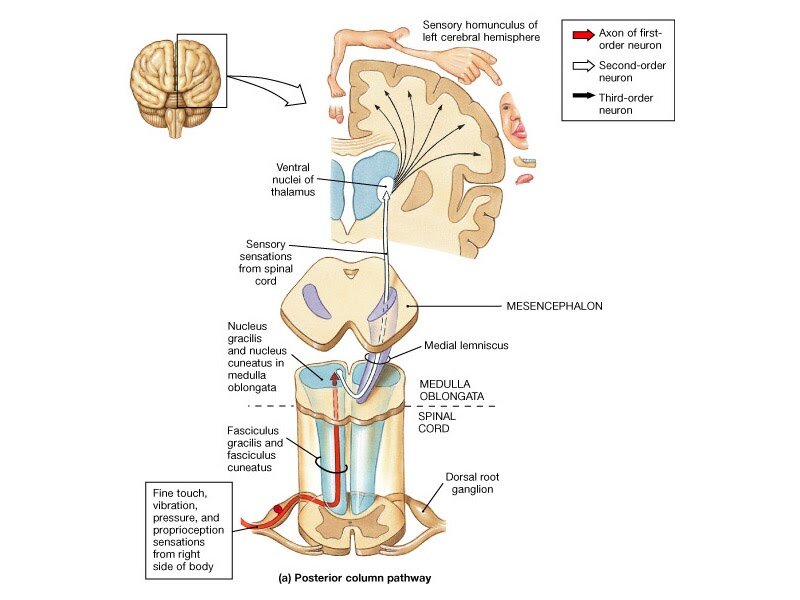
Spinothalamic Tract Neurons
Spinothalamic Tract Neurons, in Deep Dorsal Horn, Wide Dynamic Range Neurons and P-DTR. The principal ascending pathways for pain (e.g., spinothalamic and trigeminothalamic tracts) originate partly within deep or most ventral layers (IV-VI of Rexed) of the dorsal horn of the spinal cord and medulla, wherein neurons receive synaptic input from primary afferent neurons that supply nociceptors in tissue. These second-order neurons within the deep dorsal horn are mainly wide dynamic range (WDR) neurons and to a lesser extent nociceptive-specific (NS) neurons, and these two types of neurons have functional significance for processing both exteroreceptive and interoreceptive information associated with pain (Willis 1985; Price and Dubner 1977; Dubner et al. 1986).
Wide Dynamic Range (WDR) Neurons. WDR neurons are present in both superficial and deep layers of the dorsal horn (Willis 1985; Price and Dubner 1977). However, NS neurons occur in highest percentages in the superficial layers (layers I–II), and the deep layers contain mostly WDR neurons. Although they receive synaptic input from primary A-delta and C-nociceptive afferent neurons innervating cutaneous, visceral, muscular, or other tissues, they are classified on the basis of their responses to cutaneous stimuli (Willis 1985; Price and Dubner 1977). WDR neurons have several distinct and defining characteristics. First, they respond differentially over a broad range of stimulus intensity, extending from very gentle to distinctly painful levels of stimulation; noxious stimuli delivered to the most sensitive portion of their receptive fields evoke a higher impulse frequency than any form of innocuous stimulation. Second, based on multiple tests, WDR neurons also receive synaptic input from A-beta primary afferent neurons that supply sensitive mechanoreceptors in the skin (Willis 1985; Price and Dubner 1977; Price et al. 1976, 1978).
The demonstration of A-beta input prior to testing with nociceptive stimuli shows that WDR neurons in layer I are not misidentified nociceptive specific, or neurons that respond mainly to heat, pinch, and cold, as has been claimed previously (Craig 2003). WDR neurons have a very distinct receptive field organization that contains a central cutaneous zone differentially responsive to non-noxious and noxious stimuli, and a larger surrounding zone that responds mainly to nociceptive stimuli. This receptive field organization provides a critical basis whereby populations of WDR neurons could encode the distinction between nonnoxious and noxious stimulation. Multiple lines of evidence show that, in comparison to nonnoxious stimuli, noxious stimuli activate higher impulse frequencies and a larger number of WDR neurons (Price and Dubner 1977; Dubner et al. 1986; Price et al. 1976, 1978; Coghill et al. 1993). Consideration of the number of neurons activated is important in explaining encoding mechanisms because populations are what respond during non-noxious and noxious stimulations.
Beyond these defining characteristics, several features of WDR neurons are highly consistent with their role in exteroreceptive pain. First, they respond in a graded fashion to several forms of noxious stimuli that reflect contact of external objects with the skin. To a major extent, their responses to these stimuli represent characteristics of the objects themselves. Their impulse frequency increases with sharper or hotter objects (above about 45 _ C) and in a manner that closely parallels psychophysical responses (Dubner et al. 1986; Price et al. 1978; Coghill et al. 1993). Similar to pain ratings in human psychophysical experiments, their responses to contact heat stimuli in the nociceptive range (45–51 _ C) are positively accelerating power functions. WDR neurons have a very high discriminative capacity, as demonstrated in studies of WDR neurons in conscious monkeys trained in a discrimination task (Dubner et al. 1986). Consistent with both human and monkeys’ ability to detect very small shifts within the noxious temperature range, WDR neurons responded to temperature shifts as small as 0.2_ –0.4 _ C. NS neurons were much less sensitive to these small differences, and their discriminative capacity was not sufficient to account for psychophysical discriminations of either humans or monkeys. Deep dorsal horn WDR neurons have this discriminative capacity (Dubner et al. 1986). This feature of WDR neurons shows that they are a class of neurons that are part of an ascending exteroreceptive pathway that is critical for discrimination of small differences in temperature of external objects. The functional role of such refined discrimination is related to the fact that mammals actually utilize refined nociceptive discrimination to acquire information about properties of external objects. A monkey picking berries out of a thorny bush or an animal stalking prey across hot rocks pursues these tasks in order to eat. The animal must carefully weigh the painfulness of thorns or hot rocks against its need for food. In making such decisions, it must assess whether different objects in the environment or movements in different directions will result in more or less pain. This high discriminative capacity of the pain system also has an extremely important implication in pain measurement, because it suggests that there are many more discriminative levels of pain than would be implied by simple category scales or numerical rating scales.
A second reason that WDR neurons are involved in exteroreceptive pain is that they are somatotopically organized within the dorsal horn, thereby providing the basis for locating the external source of contact between objects and the body (Price and Dubner 1977; Price et al. 1976, 1978; Yokota and Nishikawa 1980; Yokota 1985).
Their somatotopic organization is a relatively unrecognized feature, and it has recently been claimed that they have only large receptive fields and, therefore, do not display somatotopic organization (Craig 2003). Yet their receptive field sizes range from very small (< 2 cm2), to medium (e.g., a portion of a foot or part of one trigeminal division) to large (larger than the foot or one trigeminal division) in monkeys (Willis 1985; Price and Dubner 1977; Price et al. 1976, 1978), and they are even smaller in cats (Yokota and Nishikawa 1980; Yokota 1985). Moreover, they are somatotopically organized in mediolateral fashion within the trigeminal (Price et al. 1976; Yokota and Nishikawa 1980; Yokota 1985) and spinal dorsal horns (Willis 1985).
A third reason that WDR neurons are involved in exteroreceptive pain is that their responses can distinguish between different forms of externally applied somatic stimulation, including the distinction between tactile stimuli and noxious stimuli. Monkey WDR spinothalamic tract neurons consistently increase their firing frequency (e.g., from below 10 Hz to 15–20 Hz) when a camel-hair brush is dragged gently across the glabrous skin of the foot (Price et al. 1978). They continue to respond at about this frequency for more than 20 s, and as long as 56 s after termination of this stimulus. This phenomenon parallels tactile after-sensations in humans evoked by the same type of stimulus (Melzack and Eisenberg 1968). For example, both human tactile after-sensations and WDR neuron after-responses abruptly terminate when the affected skin is rubbed (Price et al. 1978; Melzack and Eisenberg 1968). Since WDR neurons are the only class of spinal cord neurons that respond with tactile-after-responses, these responses are likely to be sufficient for this form of tactile sensation. Yet the same WDR neurons inevitably respond with a higher impulse frequency to noxious stimulation of the most sensitive portion of their receptive fields (Price and Dubner 1977; Price et al. 1976, 1978; Coghill et al. 1993). Thus, WDR neurons respond differentially to several forms of somatosensory stimulation, particularly when population factors are considered as factors in encoding (Coghill et al. 1993). They encode nociceptive intensity and the distinction between non-noxious and noxious stimuli with exquisite precision (Price and Dubner 1977; Coghill et al. 1993).
The method of Proprioceptive Deep Tendon Reflex (P-DTR) was founded and developed by Dr. Jose Palomar and has been successfully used in clinical practice for several years. P-DTR is the first neurological manual therapy based on neurology, neurophysiology, biomechanics and basics of Applied Kinesiology. P-DTR is a neurological, reflexogenic system, which efficiently treats a wide spectrum of functional problems and solves musculoskeletal, gastrointestinal, hormonal, chemical and emotional dysfunctions. Dysfunction is a physiological and reflexory disorder of the internal organs, which in most cases has a compensatory character. The main goal of the P-DTR treatment is to restore optimal reflexive activity of the nervous system to stimulus. This includes its motor and gland response, which would result in no symptoms of pain or discomfort perceived by the client, optimal range of motion and accurate appropriate adaptation to the conditions of the external environment. In other words, Neurological Health restores this way.
The types of manual afferent inputs (stimuli) that are used can be produced in a variety of ways including light swiping (to stimulate the receptors of touch), local stretching (to stimulate Golgi receptors), deep pressure (Pacini receptors) and many more. Today, P-DTR works with most of the exteroreceptors, interoreceptors and proprioceptors that form the afferent input to the CNS.
The physiological explanation of this method is logical – each type of receptor (for example: Golgi, Pacini, vibration, nociceptors etc.) is stimulated and when the threshold for that receptor has been exceeded by the amount of stimuli, the stimuli are converted into electrical impulses. These electrical impulses form the afferent information that reaches the CNS with each type of input being relayed along their respective pathways. The CNS receives this information, interprets it and makes a motor or gland response based on the synthesis of all the information it has received. For example, the sensation of PAIN. It is synthesized directly in the brain and is a complex product of the information from the nociceptive, proprioceptive and exteroceptive systems. Put simply, the sensation of pain would be the interpretation of the brain based on a complex integration of information from variety of different sources, as such Wide Dynamic Range Neurons and Non Specific Neurons being one of those sources.